Today, November 14th, is the anniversary of the National Childhood Vaccine Injury Act (NCVIA).
NCVIA MUST be amended to protect American children and ensure that liability is placed where it belongs: on the vaccine manufacturer. We are asking Congress to make these common sense amendments to NCVIA:
As a matter of informed consent, prior to vaccination, vaccine recipients must be informed of, and acknowledge their understanding of, their legal rights regarding any vaccine-related injury or death associated with the administration of a vaccine.
Retribution and discrimination based on an individual’s vaccination status shall be prohibited.
Coercion and Undue Influence to alter an individual's vaccination choice shall be prohibited.
Individuals may bring a civil action against the vaccine manufacturer for vaccine-related injury or death if the Secretary fails to fulfill obligations under 42 U.S.C. §300aa–-27. (FYI: The obligations under this section have NEVER been fulfilled.)
Hit the button below to send a message to your Congressmen. Tell them it is time to protect American children and put liability where it belongs: on vaccine manufacturers.
Read more about the history of NCVIA and its catastrophic effects below.
In 1986, Congress passed the National Childhood Vaccine Injury Act along with the Vaccine Injury Compensation Program (VICP) “after lawsuits against vaccine companies and health care providers threatened to cause vaccine shortages and reduce U.S. vaccination rates.”
Pharmaceutical lobbyists used fear to convince legislators that the industry’s inability to continue to pay compensation for the injuries that resulted from the use of their products would reduce vaccination rates and result in a resurgence in childhood illnesses. Instead of demanding they make safer vaccines, Congress gave them a get-out-of-jail-free-card and removed all product liability.
The passage of NCVIA in 1986 helped set the stage for the unbelievable nightmare of the last two years. Interestingly, some of the major players on the scene today were involved with its inception. For instance, Dr. Anthony Fauci was already installed at the National Institute of Allergy and Infectious Disease (NIAID).
NCVIA also laid the groundwork in eliminating any incentive for supporting readily available early treatment protocols and naturally acquired immunity to infectious disease. Drug manufacturers were given a cash cow with no incentive for product safety, a guaranteed consumer base with the introduction of school vaccination requirements, and a deceptive perception of vaccine safety with the elimination of one of the most essential market indicators of product safety - litigation. Insidiously, vaccination became the “only” way to prevent disease.
Not surprisingly, Health and Human Services (HHS) has not complied with the few safeguards that were included in NCVIA, specifically 42 U.S.C. §300aa–27 which requires:
(a)General rule
(2) make or assure improvements in, and otherwise use the authorities of the Secretary with respect to, the licensing, manufacturing, processing, testing, labeling, warning, use instructions, distribution, storage, administration, field surveillance, adverse reaction reporting, and recall of reactogenic lots or batches, of vaccines, and research on vaccines, in order to reduce the risks of adverse reactions to vaccines.
(c) Report
Within 2 years after December 22, 1987, and periodically thereafter, the Secretary shall prepare and transmit to the Committee on Energy and Commerce of the House of Representatives and the Committee on Labor and Human Resources of the Senate a report describing the actions taken pursuant to subsection (a) during the preceding 2-year period.
How has the Secretary of Health and Human Services made improvements in adverse event reporting according to 42 U.S.C. §300aa–27(a)(2)?
In 2011, the CDC commissioned Harvard Pilgrim HMO to conduct a study of the Vaccine Adverse Event Reporting System (VAERS) which concluded:
“Likewise, fewer than 1% of vaccine adverse events are reported. Low reporting rates preclude or slow the identification of “problem” drugs and vaccines that endanger public health. New surveillance methods for drug and vaccine adverse effects are needed.”
The study authors noted:
“Unfortunately, there was never an opportunity to perform system performance assessments because the necessary CDC contacts were no longer available and the CDC consultants responsible for receiving data were no longer responsive to our multiple requests to proceed with testing and evaluation.”
No improvements have been made to VAERS since its inception, so it is safe to assume that the adverse event reporting rate is still less than 1%.
Did the Secretary of HHS submit reports to Congress every two years describing the actions taken pursuant to 42 U.S.C. §300aa–27(c), including improvements in adverse event reporting?
In 2018, the Informed Consent Action Network (ICAN) sued HHS to produce the reports that should have been submitted biennially since 1989. On July 6, 2018, a judge issued a stipulated order which acknowledges that there are no such reports.
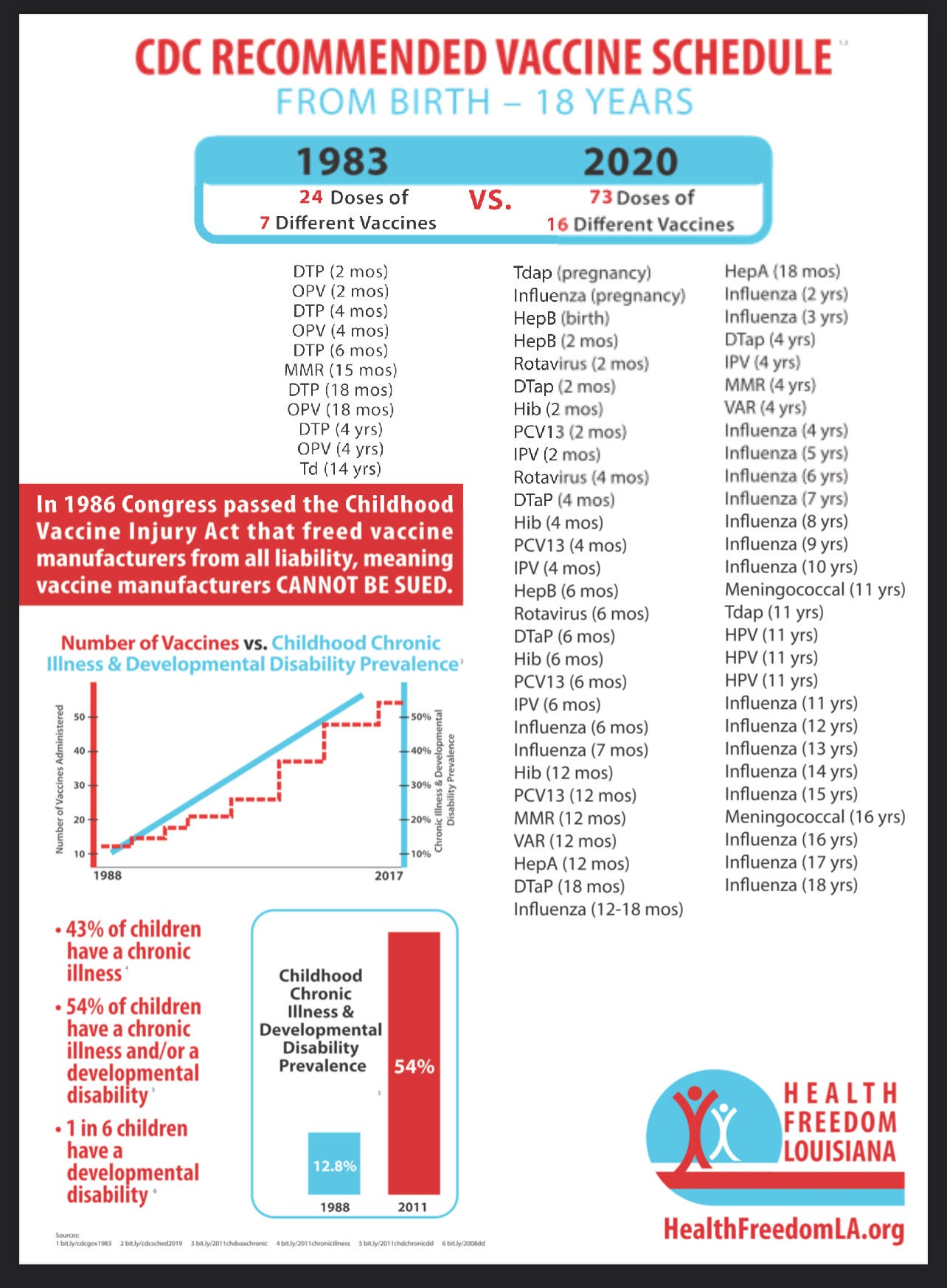
Since the inception of NCVIA in 1986, the childhood schedule has grown exponentially, going from 24 doses of seven different vaccines to 73 doses of sixteen different vaccines today.
ACIP has voted to add COVID-19 shots to the pediatric schedule, and CDC has announced that change will be made in February. If covid vaccines are added to the childhood schedule, the number of doses could increase by at least 18 doses, and likely far more, based on the number of doses and boosters the CDC has already recommended.
HHS has had 36 years to comply with the requirements of NCVIA. On what do we base the assumption of “safety” if adverse event reporting is estimated to be less than 1% and there have been no biennial reports evaluating vaccine improvements submitted to Congress for the last 36 years as is required by federal law? Congress recognized in 1986 that improvements needed to be made in the vaccine program, which was on the verge of collapse because of the number and severity of claims against vaccine manufacturers.
It is time to place the responsibility for product safety squarely where it belongs: on vaccine manufacturers. These drugs are either safe or they are not. If they are safe, there should be no liability shield. If the drugs are not safe, they should be removed from the market. The only true test of safety is product liability. If vaccine products were truly safe and effective, individuals would choose them, no mandates, coercion, or undue influence (which all violate human rights declarations) required.
Make pharma liable again.
Suggested amendments to 42 U.S.C. §300aa–1:
(B) As a matter of informed consent, prior to vaccination, recipients must be informed of, and acknowledge their understanding of, their legal rights regarding any vaccine-related injury or death associated with the administration of a vaccine.
(C) Retribution and discrimination based on an individual’s vaccination status shall be prohibited.
(D) Coercion and Undue Influence to alter an individual's vaccination choice shall be prohibited.
Suggested amendment to 42 U.S.C. §300aa–11(a):
(11) Individuals may bring a civil action for vaccine-related injury or death against the product manufacturer if the Secretary fails to fulfill obligations under 42 U.S.C. §300aa–-27. (FYI: The obligations under this section have NEVER been fulfilled.)
If you haven’t already, send a message to your Louisiana congressmen, and tell them it is time to protect American children and Make Pharma Liable Again.
Let’s celebrate next year’s anniversary with liability where it belongs: on vaccine manufacturers.